
Strengthens and Improves Your Overall Well-being
Support patients undergoing rehabilitation*
Improves motor and cognitive functions*
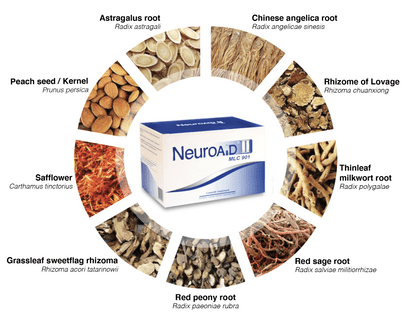
About NeuroAiD™II
NeuroAiD™II is scientifically proven to be effective in post stroke recovery to regain bodily functions after a stroke.
NeuroAiD™II supports patients undergoing rehabilitation*
How to take NeuroAiD™II?
2 capsules, 3 times a day orally*
Recommended for a 3-month period*
NeuroAiD™II is scientifically researched
Pharmacology and pre-clinical studies (made on cells cultures and animal models) have been conducted to determine the mechanisms of action of NeuroAiD, confirming the multimodal approach of the product.
Studies on NeuroAiD were led by medical experts, and study findings have been presented at international conferences and published in recognised journals.
More than 45 scientific papers have been published and the research remains active with ongoing trials and collaborations.
How To Buy NeuroAiD™II Near You
Call Neurocare Pharma LTD
Monday – Friday from 8:00 to 16:00
Folake: +234 802 384 1155
Piper: +234 905 640 1384
Faidat: +234 908 115 5201
Email: info@neurocarepharma.com
Contact Us
Get in touch
Thanks for your interest in NeuroAiD™II. To send us a message please fill in the form and we will be in touch with you soon
Send us a message
References:
- Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol 18;417-418. doi.org/10.1016/S1474-4422(19)30030-4
- Centers for Disease Control and Prevention. About Stroke. January 2020. (Accessed on June 8th 2020). https://www.cdc.gov/stroke/about.htm
- Cramer SC, Sur M, Dobkin BH et al. Harnessing neuroplasticity for clinical applications. Brains 2011;134:1591-1609. doi: 10.1093/brain/awr039.
- Heurteaux C, Gandin C, Borsotto M et al. Neuroprotective and neuroproliferative activities of NeuroAiD (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology 2010; 58(7): 987 – 1001; doi: 10.1016/j.neuropharm.2010.01.001
- Suwanwela NC, Chen CLH, Lee CF et al. Effect of combined treatment with MLC601 (NeuroAiDTM) and rehabilitation on post-stroke recovery: The CHIMES and CHIMES-E studies.

